Chirality: How chemical isomerism affects drug treatment
- Neil Sardesai

- Nov 16, 2019
- 5 min read
Updated: Nov 8, 2020
To understand the use of chirality in drug therapy, one first must understand what chirality is and how it describes how atoms in a molecule are arranged. To do this, I will first explain what optical isomerism is. Optical isomerism is a type of stereoisomerism. Stereoisomerism is where two compounds have the same molecular and chemical structure as each other but a different 3D spatial arrangement of their atoms. Optical isomerism is a subset of stereoisomerism, where the two compounds also have non-superimposable mirror images. This helps explain what chirality is, as the word "chirality" is derived from the Greek word for hand "χειρ". Like your left and right hands, which are mirror images of each other, these molecules can't be superimposed onto each other. This pair of molecules that are mirror images of each other are usually called enantiomers, however, in the context of medicine, we can also call them chiral drugs.

The classification of these enantiomers can then be further divided. One property of enantiomers, also known as optical isomers, is that they rotate the plane of polarised light that hits the molecules. If the enantiomer rotates the plane of polarised light to the right then they are classified as dextrorotatory, whereas if the enantiomer rotates the plane of polarised light to the left, we classify it as laevorotatory. These terms are often shortened to (+) for dextrorotatory (when the polarised light is rotated to the right) and (-) for laevorotatory (when the polarised light is rotated to the left). We can also call the molecules 'right-handed' and 'left-handed', depending on which way the plane of polarised light is rotated.
I'm sure by this point that you're probably wondering how this relates to the biochemistry behind drug treatment. This is because, in general, when chemical reactions are carried out (including those to manufacture medicines), a racemic mixture forms. A racemic mixture is one which contains equal proportions of (+) and (-). While it may seem that this is harmless, as the two compounds are mirror images of each other, each enantiomer actually has different functional properties! As such, the two different enantiomers have different effects on the body.
The impact of chirality is evident both in the way that natural molecules interact with our body, as well as in the impact of artificial molecules such as medicines. For example, the human body can metabolise right-handed glucose (+) but cannot metabolise left-handed glucose (-). This is due to the fact that the enzymes we have in the body that break down glucose are chiral and, as such, can only act on right-handed sugar.
Nonetheless, the chiral properties of molecules can also have devastating effects on the human body. This is because, while one enantiomer in the racemic mixture might have the desired effect and cure the disease, the other may be toxic!
The Softenon disaster of the 1950s underlined the issue of chiral drugs clearly. Only after it occurred did the pharmaceutical industry realise how devastating the consequences of mixing enantiomers could be. In the 1950s, a West German pharmaceutical company developed thalidomide, a sedative and hypnotic drug which was also used to treat nausea and gastritis. Once it became approved for over the counter sale in 1957, it also became popular to treat morning sickness. Nevertheless, this proved to have disastrous effects on unborn foetuses. Tragically, between 1957-1961, 10,000 infants who had been exposed to thalidomide before birth were born with phocomelia. Phocomelia is a congenital deformity where, in rare cases, the limbs of the baby can be so short that the hands and feet are located very close to the body. As a result, the use of thalidomide was banned in many countries by 1961.
Further research on thalidomide has shown that its chiral nature led to these catastrophic events. This is because, while the right-handed version of the drug worked as a sedative, the left-handed version was a teratogen (a drug which harms the foetus in the womb), leading the limb deformation.
Before the Softenon disaster, drug regulation was minimal. Nevertheless, this catastrophe helped catalyse a change in drug regulation, with countries such as the UK and the USA beginning to regulate the industry. Today, it is well understood that enantiomers must be thought of as separate molecules and not different forms of the same substance. As such, while drugs that consist of these racemic mixtures are not prohibited, it is now law that pharmaceutical companies must test all enantiomers of the chiral drug before it can be introduced to the market.
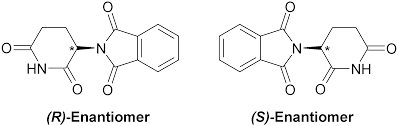
The uses of thalidomide are extremely limited. This is because, while we can isolate the right-handed form of the substance (the one that worked as intended), once injected into the body, the liver secretes an enzyme that converts the right-handed form into the left-handed molecule. As such, the harmful side effects still exist.
Rather surprisingly, thalidomide is currently approved for sale. This is on account of the fact that researchers have discovered that the greatest risk of thalidomide-induced birth defects is during the first 2 months of pregnancy. Furthermore, as the patent on thalidomide has now expired, any company can produce it, making it incredibly economical to do so. As such, due to the drug's low cost, it is experimentally used across the world to treat diseases ranging from cancer to AIDS. It is also licensed as a treatment of ENL (an inflammatory complication of leprosy). Understandably, survivors from its use in the 1950s and 1960s are concerned about its reintroduction. This is because many women don't know that they are pregnant within the first few weeks of pregnancy, but also because the drug can impact non-pregnant individuals as well, including leading to permanent nerve damage.

As evident by the above information, the separation of enantiomers is crucial in the pharmaceutical industry. Chiral separation also helps reduce the dosage of the medicine required, thus reducing its cost. While there are many techniques to separate the isomers in a racemic compound, the most common methods are to either use chemical reactions such as an acid-base reaction or to use HPLC (high-performance liquid chromatography).
Chiral drugs are incredibly important in modern-day medicine. Indeed, 56% of the drugs currently used in pharmaceuticals are chiral molecules. However, care must be taken to ensure that all enantiomers of the drug are safe. As such, I also would say that it is vital to promote chiral separation when developing new racemic drugs. Furthermore, I personally do not support the current use of thalidomide as, while its low cost allows the drug to be available to many more people than a more expensive drug would be, thalidomide clearly has a track record of being an unsafe drug.
Sources:



Comments